Can precise genetic edits save millions of vulnerable farmers from climate catastrophe? The answer lies at the intersection of cutting-edge biotechnology, food security imperatives, and the urgent need for equitable innovation.

The Global South faces an existential paradox. Home to 475 million smallholder farmers producing most of the world’s food, these regions are simultaneously the most vulnerable to climate change’s devastating impacts. Rising temperatures, erratic rainfall, and intensifying droughts threaten harvests across Sub-Saharan Africa, South Asia, and Latin America—precisely where food security remains precarious. Yet an emerging technology—genome editing through CRISPR—promises transformative possibilities for developing climate-resilient crops faster than conventional breeding could ever achieve. But realizing this promise requires far more than scientific innovation; it demands thoughtful policy, equitable access frameworks, and inclusive decision-making that centers smallholder farmers, not corporate interests.
Key Highlights
- Speed to Impact: CRISPR-based genome editing can develop climate-resilient crops in 3-5 years versus 10-15 years through traditional breeding, addressing urgent climate adaptation needs
- Proven Climate Adaptations: Genome-edited wheat and rice varieties demonstrate up to 20% yield increases under drought and heat stress through enhanced drought tolerance genes like ARGOS8 and AREB1
- Equity Crisis: Without deliberate inclusive policy frameworks, the technology risks deepening farmer inequality—patents and licensing costs could restrict access primarily to large-scale commercial operations rather than smallholders
- Regulatory Divergence: Africa, Asia, and Latin America are adopting adaptive frameworks that distinguish genome-edited crops from traditional GMOs, enabling faster deployment while the EU maintains stricter classification
- Integration Imperative: Maximum climate resilience impact requires combining CRISPR with AI-driven precision agriculture, participatory farmer research models, and robust biosafety monitoring systems
The Science Behind the Revolution: Understanding Genome Editing
Precision matters in crisis moments. When climate change threatens harvests, waiting for traditional breeding cycles is a luxury the Global South cannot afford.
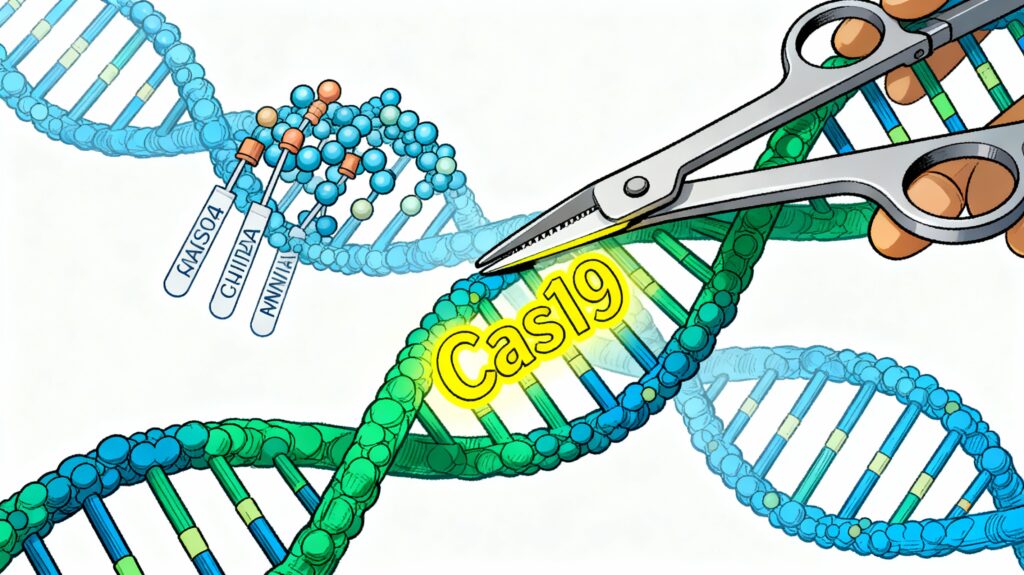
What is Genome Editing? At its core, CRISPR/Cas9 functions as molecular scissors guided by genetic GPS. The system uses a guide RNA (gRNA) to direct the Cas9 protein to a specific DNA sequence, where it makes a precise cut. Unlike earlier genetic engineering approaches that took years and often introduced unwanted genetic material, CRISPR offers unprecedented precision and speed. The edited DNA repairs itself, incorporating the desired modification—whether deleting a problematic gene, inserting beneficial traits, or fine-tuning gene expression.
Why CRISPR Matters: Traditional breeding requires identifying desirable traits, crossing plants, evaluating offspring across multiple generations, and waiting 10-15 years before a new variety reaches farmers. CRISPR compresses this timeline to 3-5 years while targeting multiple traits simultaneously. Base editors and prime editors—advanced CRISPR variants—enable even more precise modifications, including single-nucleotide substitutions without introducing foreign DNA.
This acceleration transforms the equation for climate adaptation. The traditional breeding timeline assumes stable climate conditions; CRISPR enables responsive innovation in an accelerating crisis.
India’s ICAR and international research consortia have pioneered SDN-1 and SDN-2 genome editing approaches (modification without foreign DNA introduction) that face lighter regulatory scrutiny than traditional GMOs in many countries. This regulatory flexibility, combined with precision, positions CRISPR as a pragmatic tool for climate adaptation where time is critical.
How Genome Editing Builds Climate-Resilient Crops
Climate change manifests as multiple, interconnected stresses: drought, flooding, heat, saltwater intrusion into coastal soils, and shifting pest and disease patterns. Addressing each requires different genetic strategies.
Drought Tolerance: Rewriting Water Efficiency
The Challenge: By 2030, global water stress will intensify across the Global South. In India, Pakistan, and Sub-Saharan Africa, irrigation accounts for 70-80% of freshwater use, yet agricultural water availability continues declining. Traditional crops cannot adapt quickly enough.
The CRISPR Solution: Researchers have identified and edited specific genes controlling water-use efficiency. The ARGOS8 gene, when modified in maize, reduces ethylene sensitivity and enhances drought tolerance, boosting yields by up to 15% under water-limited conditions. In rice, editing the AREB1 gene (ABA-responsive element binding protein) activates drought-responsive pathways, improving stomatal regulation and water retention.
Real-World Impact: Genome-edited rice varieties like DRR Dhan 100 and Pusa DST Rice 1 developed by India’s Directorate of Rice Research demonstrate these principles at scale, with field trials showing enhanced resilience in India’s water-stressed regions.
Heat Resilience: Crops That Survive Extremes
Temperature increases threaten staple crops worldwide. Wheat productivity declines 5-7% for every 1°C rise above optimal temperatures. Yet editing transcription factors like TaDREB2 and TaERF3 in wheat enables better thermal regulation and recovery from heat stress.
Pest and Disease Resistance: Breaking the Pesticide Treadmill
Emerging pathogens increasingly threaten crops as climates change. Genome editing can introduce durable resistance faster than conventional breeding. Researchers in Burkina Faso are piloting genome-edited rice resistant to bacterial blight—a devastating pathogen spreading through warming West African regions. Similarly, cassava varieties edited for enhanced disease resistance are advancing through trials in Sub-Saharan Africa, offering smallholders reduced dependency on expensive fungicides and insecticides.
Nutritional Enhancement: Feeding, Not Just Sustaining
Climate resilience alone addresses survival; nutrition addresses flourishing. In India and Sub-Saharan Africa, micronutrient malnutrition affects nearly 2 billion people. Genome-edited millets with enhanced iron and zinc content—crops naturally suited to dryland regions—represent a dual opportunity: climate adaptation and improved nutrition.
Integration with Precision Agriculture and AI
The synergy multiplier emerges when CRISPR-edited varieties are combined with data-driven farming technologies. AI-powered systems using satellite imagery, IoT soil sensors, and machine learning can monitor genome-edited crop performance in real-time, detect early stress indicators, and recommend precision interventions. In precision agriculture platforms, AI algorithms analyze weather patterns, soil conditions, and plant genetics simultaneously, enabling farmers to make decisions that maximize resilience while minimizing inputs.
This integration creates a closed-loop system: genome editing provides genetic adaptation capacity; precision agriculture maximizes that capacity through data-driven optimization.
Sustainability and Resource Efficiency: Beyond Higher Yields
Climate resilience agriculture must also regenerate natural systems, not merely extract from them.
- Reduced Agrochemical Dependency: Traditional breeding often maintains existing pesticide and fertilizer needs. CRISPR enables trait stacking—combining drought tolerance, pest resistance, and nitrogen efficiency in single varieties. In Southeast Asia, genome-edited rice varieties engineered for enhanced nitrogen-use efficiency reduce fertilizer requirements by 20-30%, cutting input costs for smallholder farmers while reducing agricultural runoff and water pollution.
- Soil and Water Conservation: Crops with modified root architecture (improved penetration and nutrient uptake in marginal soils) enable cultivation on land currently considered unsuitable. In coastal Gujarat and Rajasthan, salt-tolerant genome-edited crops could transform saline soils into productive farmland, reducing pressure on freshwater irrigation and preserving ecosystems.
- Biodiversity Enhancement, Not Erosion: Unlike industrial monoculture approaches, participatory genome editing can improve local crop varieties—preserving genetic diversity while enhancing resilience. When farmers collaborate with scientists to edit their own landraces, the result is adapted crops rooted in local agroecology rather than imported uniformity.
The Equity Crisis: Will Smallholders Benefit or Get Left Behind?
This is where noble scientific intentions confront harsh economic realities.
The Access Paradox: CRISPR’s core technology is relatively inexpensive—a stark difference from 1980s genetic engineering. Yet the patent landscape surrounding CRISPR applications is dense and fragmented. Companies like Corteva, DuPont Pioneer, and BASF hold multiple patents on specific editing methods, target genes, and trait combinations. For publicly-funded research institutions in the Global South, navigating this IP maze requires expensive legal expertise and licensing negotiations.
The Smallholder Reality: 475 million smallholder farmers produce 80% of food in Sub-Saharan Africa and South Asia. Most operate on less than one hectare, with average incomes under $2 per day. They cannot afford high-cost seeds or annual licensing fees. Yet without deliberate policy intervention, CRISPR-improved varieties will be available primarily through commercial seed companies serving large-scale farmers—precisely those least vulnerable to climate impacts. prism.sustainability-directory
Gender Dimensions: Women farmers, who comprise 40-50% of agricultural labor in the Global South, often lack land ownership, extension service access, and participation in technology adoption decisions. Without intentional inclusion mechanisms, genome-edited crops will deepen existing gender inequalities.
“Seed Sovereignty” Concerns: Traditional farming practice—saving, replanting, and exchanging seeds—has enabled farmer agency and genetic diversity for millennia. Corporate-controlled, patent-protected seeds break this cycle, creating annual dependency on purchases. India’s Biotech-KISAN program attempts to preserve this autonomy through public-sector variety development and farmer-centered research hubs.
Policy Solutions for Equitable Access
India’s “One Nation, One License” Model: India is exploring pooled licensing arrangements where the government negotiates master licenses for CRISPR toolkits, granting access to both public and private institutions under shared terms. This reduces individual institutional costs while maintaining innovation incentives.
Public Bio-Commons Framework: Rather than relying solely on IP protection, some organizations are placing edited genes into open-source commons. The Bio-commons model—adopted in parts of Sub-Saharan Africa and Asia—publishes genetic modifications, breeding protocols, and varieties publicly, enabling any researcher or farmer to use them freely.
Participatory Genomic Editing: The most promising equity framework involves farmers as co-designers. Farmers identify priority traits (drought tolerance, local cooking qualities, market preferences), researchers provide genome editing expertise, and improved varieties remain publicly owned. This model has been piloted in East Africa and India with positive community reception.
Humanitarian Licensing: Companies including Corteva and Pairwise have granted humanitarian licenses to international research centers like CIMMYT and IRRI, enabling development of public-good varieties for smallholders without profit extraction. This precedent, if expanded, could democratize access.
The Biosafety Reality: Off-Target Effects, Gene Flow, and Ecological Unknowns
Precision tools can still miss their targets. Responsible deployment requires honest assessment of real risks.
Off-Target Effects: CRISPR’s guide RNA occasionally directs Cas9 to non-target DNA sequences with similar sequences, causing unintended modifications. Most off-target edits are small indels (1-22 base pairs) and occur at lower rates than spontaneous mutations, but detecting them requires rigorous genome-wide screening. Advanced CRISPR variants like Cas12b offer improved specificity, yet no system guarantees zero off-target effects.
Gene Flow and Ecosystem Impact: Edited crops will breed with wild relatives in agricultural regions. Pollen from drought-tolerant rice could reach wild rice populations, potentially altering ecological balances. The risk remains modest—most edited crops lose fitness in wildtype competition—yet precaution demands post-release monitoring systems.
Risk Assessment Gaps: In the Global South, capacity for comprehensive ecological monitoring is limited by funding and expertise. Open-air CRISPR applications (directly releasing CRISPR machinery into environments) present particular concerns for uncontrolled modifications in non-target organisms. Emerging biosafety frameworks must address this frontier.
The Comparative Risk Lens: Evidence indicates CRISPR-induced off-target mutation rates are lower than conventional mutagenic breeding methods, suggesting that the technology is not uniquely risky—yet represents a paradigm shift requiring new oversight structures.
Policy and Regulatory Frameworks: Balancing Innovation with Safety
The regulatory landscape is splintering along geographic lines, creating both opportunity and chaos.
Divergent Regulatory Approaches
The EU Path: Precautionary Regulation: The European Union classifies genome-edited organisms as GMOs, triggering extensive risk assessment and public engagement requirements. This approach prioritizes transparency but slows innovation—few gene-edited crops have achieved commercial approval in Europe.
The Adaptive Global South Model: Burkina Faso, Ethiopia, Kenya, Nigeria, and India are pioneering risk-proportionality frameworks that distinguish between:
- SDN-1 (modifications without foreign DNA): Lower regulatory scrutiny
- SDN-2 (modifications with temporary foreign DNA presence): Intermediate review
- SDN-3 (permanent foreign DNA): Full GMO-level assessment
This approach recognizes that genome editing without introduced DNA presents fundamentally different risks than classical GMOs.
India’s framework, formalized through Department of Biotechnology guidelines (2022) and MoEFCC exemptions (March 2022), allows SDN-1 and SDN-2 crops to proceed with open field trials following institutional biosafety committee approval—a pathway enabling faster deployment for public-good varieties.
Recommended Policy Imperatives
1. Adaptive, Science-Driven Regulation: Regulatory frameworks should reflect actual risk profiles, not ideological resistance to genetic modification. Proportional assessment—recognizing that SDN-1 edits carry lower environmental risk—accelerates public-good applications while maintaining safety oversight.
2. Transparency and Stakeholder Engagement: Successful deployment requires building public trust through transparent communication. Kenya and Nigeria require notification of stakeholder communities before field trials—a model balancing innovation speed with democratic participation.
3. Global Harmonization of Standards: Divergent regulations create trade barriers and regulatory arbitrage risks. International bodies like the Cartagena Protocol should develop guidance recognizing distinction between gene-editing techniques rather than applying blanket GMO classifications.
4. Mandatory Post-Release Monitoring: Public investment must fund ecosystem monitoring to detect unintended gene flow, off-target ecological impacts, and performance under real-world conditions.
5. Rights-Protective Intellectual Property Policy: Governments must actively negotiate with patent holders to ensure humanitarian and public-good exceptions. India’s proposed “One Nation, One License” model demonstrates how pooled licensing can balance IP protections with equitable access.
6. Capacity Building for Global South Institutions: National agricultural research institutes in Africa, Asia, and Latin America require sustained funding and technical support to conduct genome editing research independently, rather than remaining dependent on technology transfer from corporate entities.
Real-World Trajectories: Evidence from Pilot Regions
Theory must meet practice. Where are genome-edited crops making measurable differences for vulnerable farmers?
Sub-Saharan Africa: Building Regulatory Pathways
Nigeria’s NBMA (National Biosafety Management Agency) authorized genome-editing guidelines in December 2020, recognizing it as part of the solution to food security and malnutrition. SHESTCO (Sheda Science and Technology Complex) and premier universities across Nigeria’s six geopolitical zones are now training biotechnologists capable of developing region-appropriate varieties. Burkina Faso is piloting genome-edited bacterial-blight-resistant rice—directly addressing a pathogen threatening the Sahel’s food security.
In Kenya and Ethiopia, young scientists are developing phosphorus-efficient soybeans using CRISPR, addressing widespread soil nutrient deficiency limiting yields across smallholder farms.
India: Policy Leadership and Farmer Integration
India’s commitment extends beyond research to institutional policy. The Ministry of Education’s October 2025 announcement—mandating AI and Computational Thinking curricula from Class 3 onwards, with emphasis on ethical AI deployment—signals long-term commitment to building a generation of “AI-empowered, not AI-dependent” citizens. This investment in education infrastructure complements biotechnology R&D.
Critically, India’s Biotech-KISAN program embeds farmer collaboration into research design. The program reports success improving drought-resistant varieties through participatory evaluation, with farmers in Maharashtra and Rajasthan actively involved in trait selection and field testing.
Latin America: Accelerated Breeding Innovation
MasAgro, a public-private partnership in Mexico, demonstrates how CRISPR can accelerate maize improvement without requiring wholesale replacement of traditional seed systems. New hybrids developed through collaborative breeding are disseminated through existing farmer networks, preserving cultural adoption pathways while introducing genomic innovations.
Argentina, Brazil, and Paraguay have developed flexible regulatory frameworks distinguishing CRISPR-edited crops from traditional GMOs, enabling faster field trials.
Integrating Technology: AI, Precision Agriculture, and Genome Editing
The transformation multiplies when genome editing combines with complementary technologies.
AI-Driven Crop Monitoring and Optimization
CRISPR-edited varieties represent genetic adaptation capacity; AI systems translate that capacity into realized yields. Machine learning algorithms analyzing satellite imagery, drone footage, and IoT soil sensors can detect early stress in genome-edited crops, recommend precise irrigation timing, and forecast yields with 88% accuracy. For smallholder farmers without extension service access, AI-powered advisories on smartphones provide actionable insights—which edit-enhanced variety suits this season’s weather? When to plant? When to irrigate?
Precision Phenotyping Accelerates Breeding
High-throughput phenotyping (HTP) uses computer vision and deep learning to measure plant traits at scale—leaf color, height, flowering time—enabling rapid evaluation of thousands of edited lines. In India’s ICAR centers and CIMMYT breeding programs, HTP has reduced evaluation cycles from years to months, accelerating the journey from lab to field.
Participatory AI for Inclusive Crop Improvement
The most innovative integration emerges through participatory AI models, where farmers with smartphones collect crop health data alongside scientists, creating localized datasets that train AI models specific to regional conditions. This approach respects farmer knowledge while leveraging computational power—a democratic technology model rather than extractive.
Conclusion: Responsibility, Not Inevitability
Genome editing cannot cause climate resilience; farmers, knowledge, and commitment to equity create it. CRISPR is a precision tool—powerful, but only as ethical and equitable as the policies guiding its deployment.
The evidence is compelling: genome-edited drought-tolerant rice and wheat can reach vulnerable farmers 10-12 years faster than conventional breeding, potentially preventing harvests losses affecting millions. Yet evidence equally suggests that without deliberate policy—open-source commons frameworks, participatory research models, regulatory proportionality, and negotiated IP fairness—the technology will deepen inequalities, concentrating benefits among large-scale commercial operations while marginalizing the 475 million smallholders producing most Global South food.
The moment demands proactive leadership. Governments in India, Nigeria, Kenya, Brazil, and Vietnam are pioneering pathways; their success or failure will shape whether CRISPR becomes a tool for shared prosperity or corporate concentration.
What should policymakers prioritize? Adaptive regulation enabling faster deployment while maintaining safety oversight. Public investment in capacity building, ensuring National Agricultural Research Institutes can conduct independent research. Equity-protective IP policies ensuring humanitarian licenses and public commons access. Participatory research models centering farmer agency and local knowledge.
What role should scientists play? Transparent communication about real risks and genuine possibilities. Commitment to open-access publishing, reducing knowledge monopolization. Partnerships with smallholder farmer organizations, ensuring research targets their needs rather than corporate profit margins.
What will determine success? Not the elegance of the genetic edits, but the integrity of the institutions deploying them. Climate resilience is impossible without trust, and trust emerges from inclusive decision-making where farmers’ voices are heard, smallholders’ interests are protected, and the benefits of innovation are shared rather than extracted.
The technology is ready. The science is proven. The question is whether we—as policymakers, scientists, and global citizens—have the commitment to deploy it responsibly.







+ There are no comments
Add yours