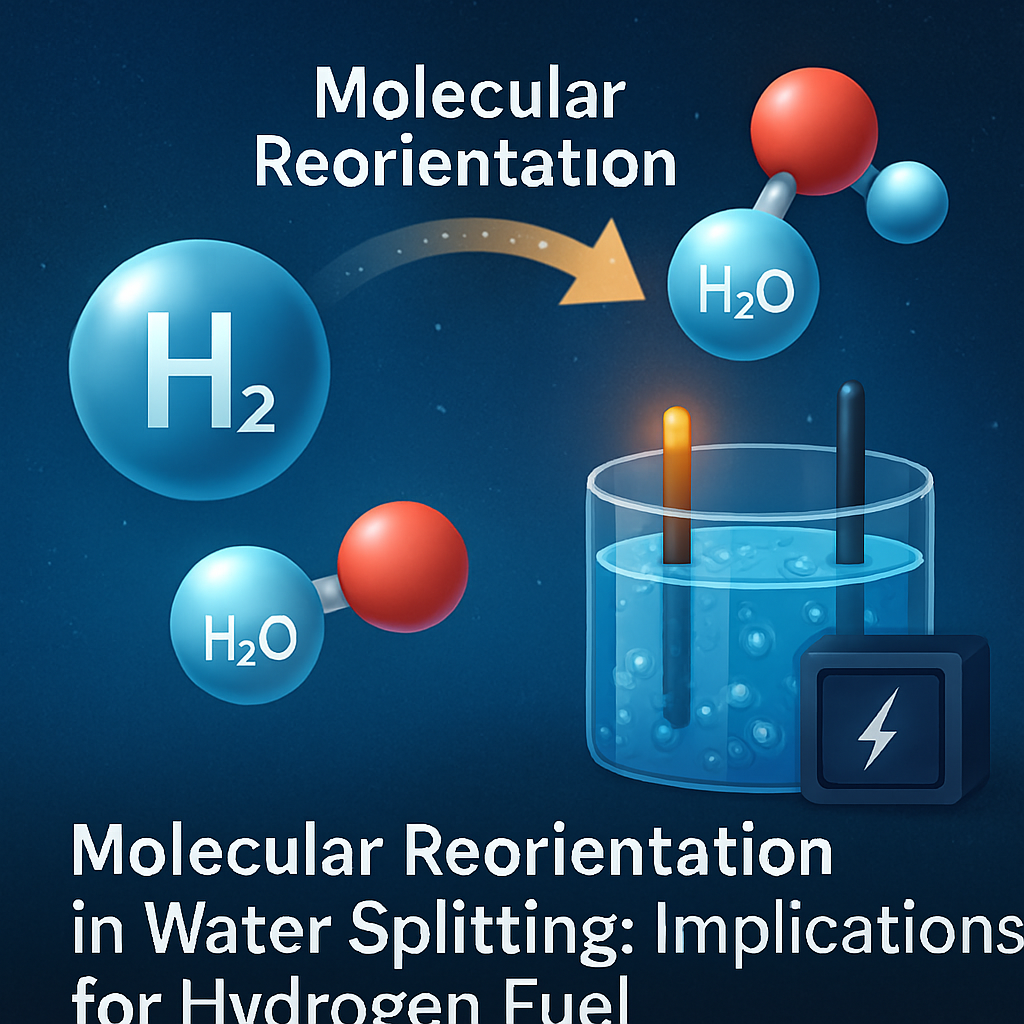
Hydrogen is considered one of the most promising clean energy sources, offering a sustainable alternative to fossil fuels. One of the most common methods for producing hydrogen fuel is water electrolysis, where water molecules (H₂O) are split into hydrogen (H₂) and oxygen (O₂) using electricity. However, researchers have long observed that the energy required for water splitting is higher than theoretical calculations predict. A recent discovery has shed light on a molecular behavior that could explain this energy discrepancy: an unexpected 180-degree flip that water molecules undergo during the splitting process. This finding could lead to more efficient hydrogen production methods, reducing costs and making hydrogen energy more viable on a large scale.
The Science Behind Water Splitting
Water splitting is a chemical reaction that separates water into its elemental components—hydrogen and oxygen. The process typically occurs in an electrolyzer, where an electric current passes through water, facilitating the breakdown of its molecules:
This reaction takes place in two half-reactions:
- Oxidation at the anode:
- Reduction at the cathode:
Despite advances in electrolysis technology, the energy consumption remains higher than expected, prompting researchers to investigate the molecular dynamics at play.
The Discovery: Molecular Flip in Water Splitting
Recent research has revealed that during the electrolysis process, water molecules experience an unanticipated 180-degree flip. This molecular reorientation consumes additional energy, which contributes to the higher-than-predicted energy demands of water splitting. Scientists believe that the flip is caused by interactions at the interface of the electrode and water molecules, influencing how the molecules bond and break apart.
Why Does This Flip Matter?
This discovery is significant for several reasons:
- Energy Efficiency: Understanding why extra energy is required for water splitting can help optimize the electrolysis process, reducing energy losses.
- Hydrogen Production Costs: A more efficient process means lower costs, making hydrogen fuel more competitive with traditional fossil fuels.
- Catalyst Development: New catalysts that minimize the molecular flip could improve overall efficiency and performance.
- Green Energy Advancement: Enhancing hydrogen production methods aligns with global efforts to transition to cleaner energy sources.
Implications for Hydrogen Fuel Production
The unexpected molecular flip presents both challenges and opportunities for hydrogen fuel production.
Challenges
- Energy Waste: The additional energy required due to the molecular flip reduces overall efficiency.
- Complexity in Catalysis: Current catalysts may not be optimized to counteract this effect, requiring further development.
- Cost of Innovation: Research into overcoming this molecular behavior requires investment in new technologies and experimental approaches.
Opportunities
- Optimized Catalysts: Scientists can design catalysts that minimize the flip, reducing energy waste.
- Improved Electrolyzers: Electrolyzer designs could be adapted to better accommodate the molecular orientation shifts.
- Broader Clean Energy Applications: More efficient hydrogen production could accelerate the adoption of hydrogen fuel in various industries, including transportation, manufacturing, and energy storage.
Potential Solutions and Future Research
Researchers are now exploring ways to mitigate the effects of the molecular flip to enhance efficiency. Some possible solutions include:
- Advanced Catalyst Design
- Developing catalysts that stabilize water molecules to prevent the flip.
- Using new materials such as transition metal oxides and perovskites.
- Electrode Surface Modifications
- Engineering electrode surfaces to alter water molecule interactions.
- Applying coatings that promote efficient bonding and dissociation.
- Adjusting Electrolysis Conditions
- Modifying voltage and current densities to optimize reaction pathways.
- Experimenting with different electrolyte compositions to influence molecular orientation.
- Quantum-Level Simulations
- Using quantum mechanics and computational simulations to understand and predict molecular behaviors in electrolysis environments.
- Developing AI-driven models to optimize water-splitting reactions.
The Future of Hydrogen Energy
Hydrogen energy has immense potential to reshape the energy landscape, providing a sustainable and clean alternative to fossil fuels. By addressing the inefficiencies in water splitting, particularly the molecular flip, we can enhance the viability of hydrogen production on a large scale.
With ongoing research and innovation, the dream of a hydrogen-powered world is becoming increasingly realistic. From powering vehicles to generating electricity, hydrogen fuel could play a crucial role in the global transition to renewable energy. By optimizing electrolysis and minimizing unnecessary energy consumption, we can unlock new possibilities for a cleaner and more sustainable future.
Conclusion
The recent discovery of an unexpected molecular flip in water splitting has provided critical insights into why the process requires more energy than theoretical models suggest. While this presents challenges, it also opens doors for innovation in catalyst design, electrolysis technology, and hydrogen fuel production. By addressing these inefficiencies, we can improve hydrogen energy’s feasibility and contribute to a greener, more sustainable world.
As researchers continue to explore this phenomenon, the future of hydrogen fuel looks brighter than ever. Investing in efficient, cost-effective hydrogen production will be a key step toward achieving global clean energy goals and reducing reliance on carbon-based fuels.






+ There are no comments
Add yours